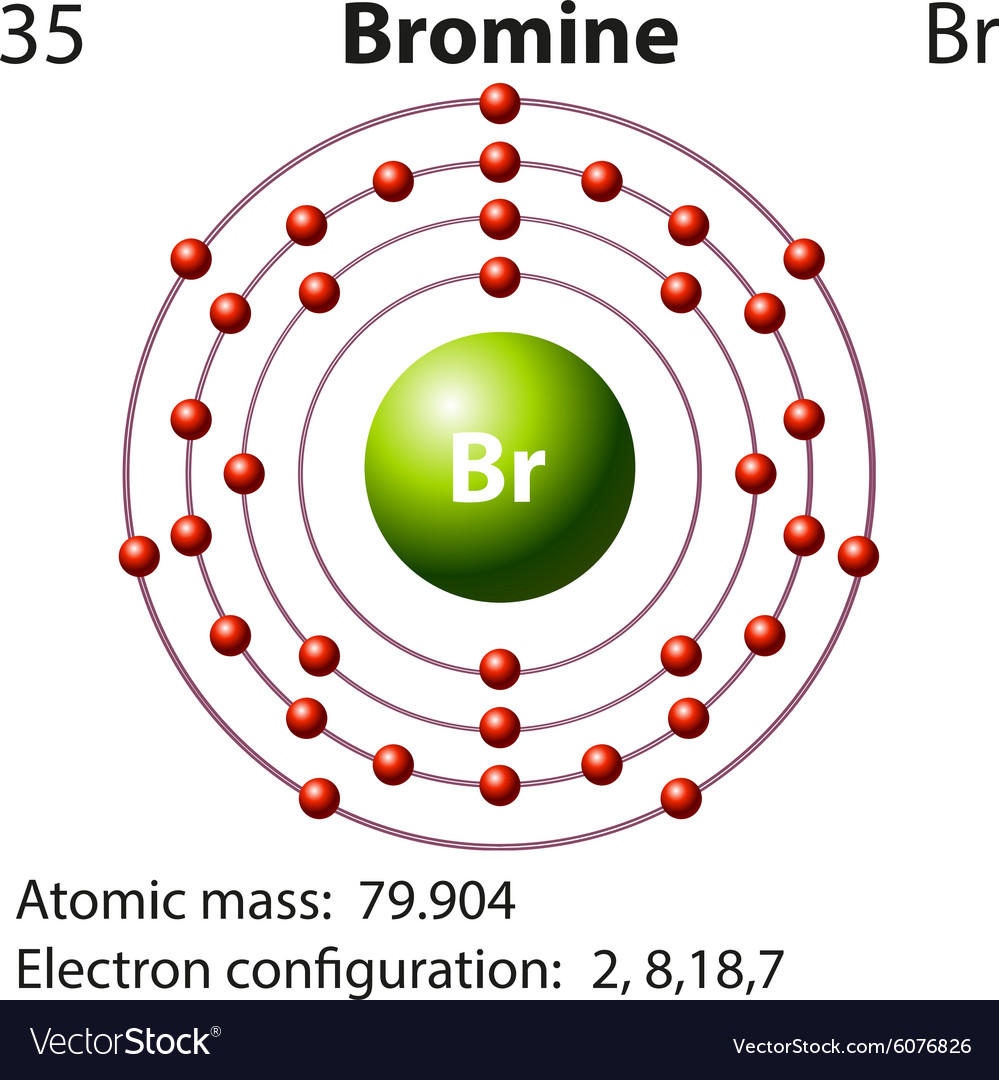
One mole of Br has mass = 79.904 g. One mole = 6.02 x 10^23 atoms. So 6.02 x 10^23 atoms have mass = 79.904 g. Mass of one atom = 79.904 / 6.02 x 10^23=1.33 x 10^-22 g. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). Note that in linear diatomic molecules, the pz orbital always points along the internuclear axis, so it has to contribute to one of the sigma bonds. I've drawn the overlaps below in the MO diagrams.
- Bromine Atom Structure
- Bromine Atomic
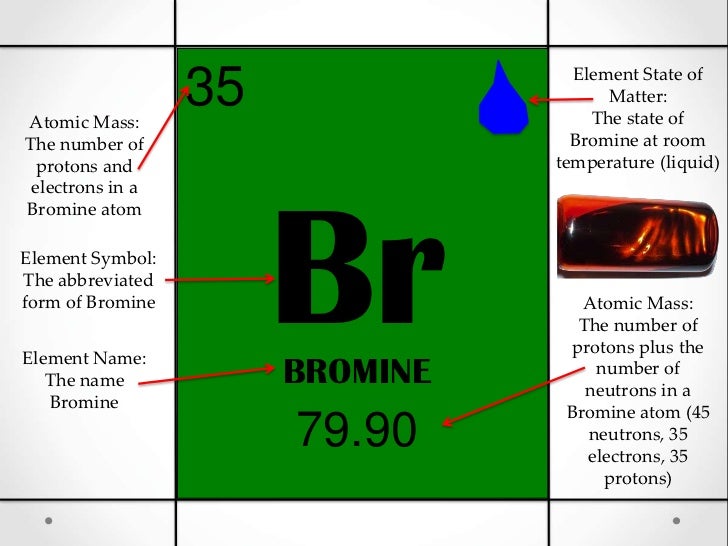
- Bromine Atomic Mass
- Bromine Atomic Weight
- Bromine Atom Charge
- Bromine Electron Configuration
MELTING POINT: −7.3°C
BOILING POINT : 59°C
DENSITY : 3.12 g/cm 3 (liq. at 20°C)
MOST COMMON IONS : Br − , BrO − , BrO 3 − , BrO 4 −
Bromine Atom Structure

Bromine Atomic
Bromine is a member of a family of elements known as halogens that are found in group 7A of the Periodic Table. Mac chrome your connection is not private. Increased heart beat cause. Bromine was discovered in 1826 in Montpellier, France, by French chemist Antoine J. Balard.
Bromine is one of two elements (the other being mercury) that is liquid at normal temperatures. As with the other halogens, bromine is very reactive, corrosive, and poisonous. Both the liquid and vapor of bromine are deep red in color. Bromine has a pungent, irritating odor that is the source of the element's name (the Greek word bromos means 'stench').
Elemental bromine is a diatomic molecule (Br 2 ). Bromine will combine with most other elements. Reaction with metallic elements leads to salts such as silver bromide (AgBr), in which the bromine atom has a −1 charge and oxidation number. Bromine forms many interesting covalent compounds as well, including two oxides: bromine (IV) oxide (BrO 2 ) and bromine (I) oxide (Br 2 O).
Bromine is produced commercially from natural brines and from sea-water either by electrolysis or with displacement by chlorine, a somewhat more reactive halogen. The concentration of bromine in seawater is approximately 67 parts per million (ppm) by weight; it is found in Earth's crust at an average level of 3 ppm.
Bromine Atomic Mass
Bromine compounds have a variety of uses. Methyl bromide (CH 3 Br) is a common agricultural soil fumigant; other bromohalocarbon compounds have been used as refrigerants and fire suppressants. Inorganic bromides are important components of photographic emulsions. Bromine reacts with liquid water to produce hypobromite ion (BrO − ), a powerful bleaching agent. There are also many dyes and pharmaceutical agents that contain bromine.
SEE ALSO Halogens .
John Michael Nicovich
Bibliography
Bromine Atomic Weight
Lide, David R., ed. (2003). The CRC Handbook of Chemistry and Physics , 84th edition. Boca Raton, FL: CRC Press.

Bromine Atom Charge
Internet Resources
Bromine Electron Configuration
Winter, Mark. 'Bromine.' The University of Sheffield and WebElements Ltd., U.K. Available from http://www.webelements.com .
